Trending...
- Pacific Harbor Line's Cliatt II Receives Black History Month Trailblazer of the Century Award - 103
- Sellvia Market Enhances Quality Screening for Marketplace Listings
- Transcure Responds to CMS Removal of 285 Inpatient-Only Procedures
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP; $750 Million Market for Suicidal Depression Market Awaits
MIAMI - Californer -- A $750 million global market for preservative-free IV ketamine is now within reach for NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), following the company's announcement that the U.S. Food and Drug Administration (FDA) has formally accepted its Abbreviated New Drug Application (ANDA) for KETAFREE™, the first known preservative-free ketamine formulation. The FDA has deemed the application "substantially complete," assigning a GDUFA target action date of July 29, 2026—a critical milestone that positions NRx for potential entry into a high-demand therapeutic market.
The FDA submission comes as independent analyst D. Boral issues a Buy rating with a $34 price target, citing NRx's maturing regulatory position, expanding revenue-generating operations, and strong clinical catalysts across its NMDA-focused drug platform.
A Transformative Solution for a Critical Mental Health Crisis
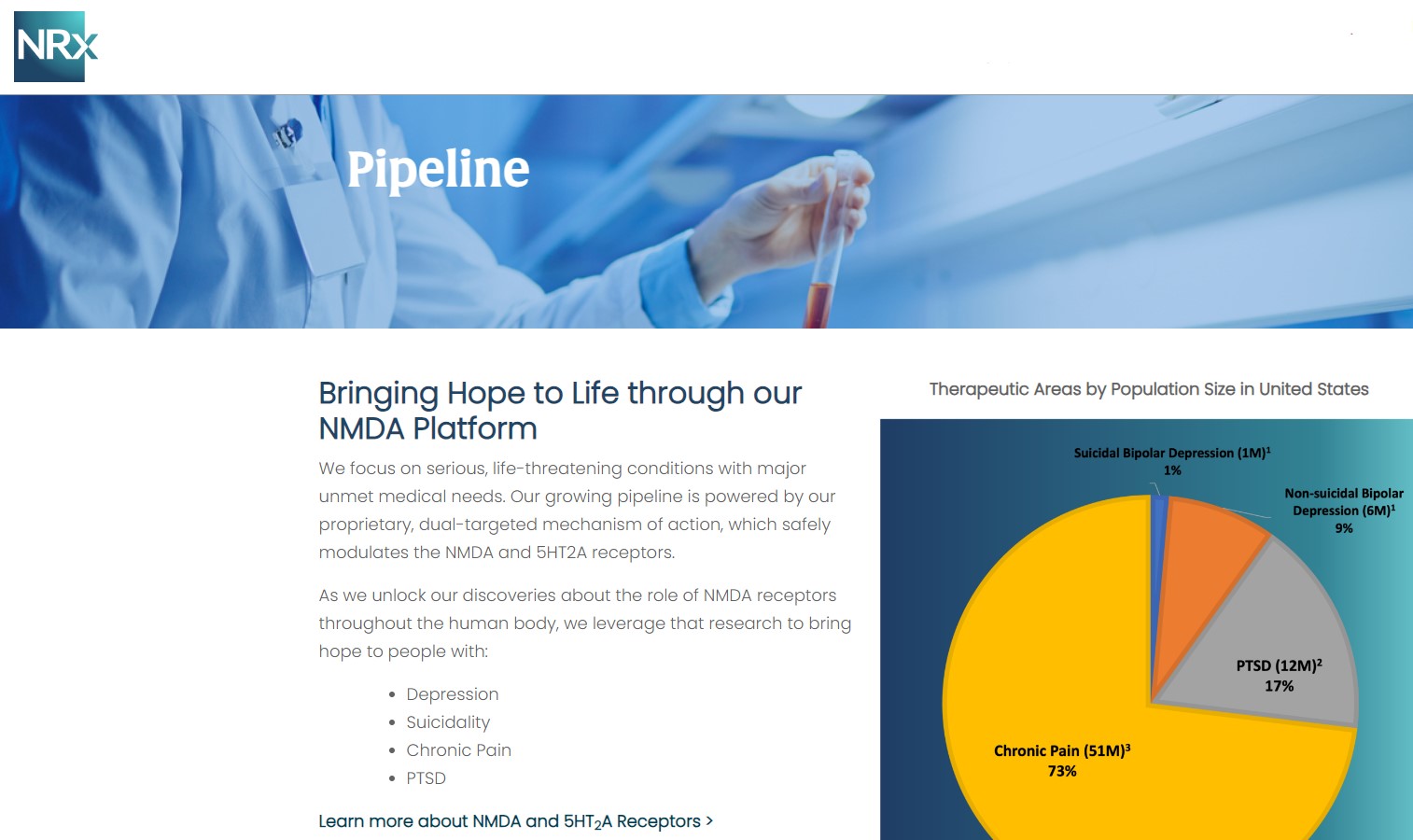
More than 13 million Americans contemplate suicide each year, according to the CDC. Current treatment options are limited, and many lack evidence of rapid antisuicidal benefit. NRx's pipeline directly targets this gap:
NRX-100 (IV Ketamine)
NRX-101 (D-Cycloserine + Lurasidone)
KETAFREE™: A Strategic ANDA Pathway and Major Competitive Advantage
More on The Californer
The global generic ketamine market is estimated at $750 million annually. Nearly all existing products contain benzethonium chloride (BZT)—a preservative banned from topical antiseptics and not recognized as safe by the FDA.
NRx's preservative-free KETAFREE™ directly addresses this safety concern.
The company has:
KETAFREE™ is strategically separate from the company's innovative NRX-100 program, enabling two simultaneous FDA pathways:
Expanding Commercial Footprint: HOPE Therapeutics Clinics
2025 marks NRx's entry into revenue-generating operations through its HOPE Therapeutics subsidiary.
The company currently operates three clinics in Florida, with six more expected by year-end, targeting treatment-resistant psychiatric conditions, chronic pain, and military/veteran mental health.
NRx also launched Florida's first deployment of the ONE-D protocol with Ampa Health, a groundbreaking, single-day treatment approach utilizing an FDA-cleared device. Peer-reviewed results of the protocol show up to 87% response and 72% remission rates, signaling a disruptive new pathway for addressing treatment-resistant depression.
Capital Secured Through July 2026—With Additional Upside from Operating Revenue
More on The Californer
NRx reports that it has secured operating capital for ongoing development through July 2026, covering the critical period leading up to the FDA's action date on the KETAFREE™ ANDA submission. With revenue now emerging from HOPE clinics and an expanding patient base, the company expects increasing non-dilutive revenue contribution.
Strategic Partnerships Strengthening Commercial Potential
NRx has partnered with Alvogen Pharmaceuticals, leveraging Alvogen's global reach to support development and commercialization of NRX-101 for suicidal bipolar depression. Additional potential indications include non-opioid chronic pain management and treatment of complicated UTIs.
A Pivotal Moment for NRXP Investors
Between its dual-path FDA strategy, real-world data advantages, strong analyst sentiment, and rapidly expanding commercial infrastructure, investors now see NRx Pharmaceuticals reaching an inflection point:
With updated clinical results, strong regulatory momentum, and multiple near-term catalysts, NRx is uniquely positioned to become a leader in next-generation therapies for suicidal depression—a market with urgent unmet need and substantial commercial potential.
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
The FDA submission comes as independent analyst D. Boral issues a Buy rating with a $34 price target, citing NRx's maturing regulatory position, expanding revenue-generating operations, and strong clinical catalysts across its NMDA-focused drug platform.
A Transformative Solution for a Critical Mental Health Crisis
More than 13 million Americans contemplate suicide each year, according to the CDC. Current treatment options are limited, and many lack evidence of rapid antisuicidal benefit. NRx's pipeline directly targets this gap:
NRX-100 (IV Ketamine)
- Awarded Fast Track Designation by the FDA for reducing suicidal ideation in depression, including bipolar depression.
- Supported by results from well-controlled NIH-sponsored studies as well as newly licensed data from French health authorities.
- Being pursued via an NDA, expected to be completed in Q4 2025, supported by real-world data from 60,000 IV ketamine patients versus 6,000 intranasal S-ketamine patients. Early analyses suggest faster onset and greater impact compared to nasal formulations.
NRX-101 (D-Cycloserine + Lurasidone)
- An investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression.
- New real-world evidence shows D-cycloserine may double the effectiveness of TMS, opening additional market opportunities in treatment-resistant depression and chronic pain.
KETAFREE™: A Strategic ANDA Pathway and Major Competitive Advantage
More on The Californer
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- From Orientation to IEP Meetings: How AI Is Helping Schools Build Stronger Communities
- Arcuri Group Announces Long‑Term Partnership with WakeMed Health & Hospitals to Deliver Situational Awareness and De‑escalation Training
- California: As Trump tears apart decades of environmental progress, Governor Newsom restores nearly 300,000 acres of habitat and cuts average permitting time to 42 days
- New Rock Music Release "Groove Train"
The global generic ketamine market is estimated at $750 million annually. Nearly all existing products contain benzethonium chloride (BZT)—a preservative banned from topical antiseptics and not recognized as safe by the FDA.
NRx's preservative-free KETAFREE™ directly addresses this safety concern.
The company has:
- Applied for KETAFREE™ as a proprietary product name
- Manufactured initial registration lots and prepared capacity for 1 million vials per month
- Filed a Citizen Petition urging removal of BZT from all U.S. ketamine products
- Positioned manufacturing entirely within the United States, aligning with national efforts to secure domestic drug supply chains
KETAFREE™ is strategically separate from the company's innovative NRX-100 program, enabling two simultaneous FDA pathways:
- ANDA approval (generic route) for rapid commercialization
- Fast Track NDA approval (innovative route) for suicidal depression—an unmet medical need with multibillion-dollar potential
Expanding Commercial Footprint: HOPE Therapeutics Clinics
2025 marks NRx's entry into revenue-generating operations through its HOPE Therapeutics subsidiary.
The company currently operates three clinics in Florida, with six more expected by year-end, targeting treatment-resistant psychiatric conditions, chronic pain, and military/veteran mental health.
NRx also launched Florida's first deployment of the ONE-D protocol with Ampa Health, a groundbreaking, single-day treatment approach utilizing an FDA-cleared device. Peer-reviewed results of the protocol show up to 87% response and 72% remission rates, signaling a disruptive new pathway for addressing treatment-resistant depression.
Capital Secured Through July 2026—With Additional Upside from Operating Revenue
More on The Californer
- JiT Home Buyers Announces Standardized Nationwide Operating Model to Strengthen Homeowner Experience
- Life Hacks of the Rich and Famous named "Best Self Help Podcast"
- Seth Neblett's Mothership Connected: Focuses on P-Funk's Women as Mothership Celebrates 50 Years
- Passive Appoints Ana Bolčević as Head of Design
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
NRx reports that it has secured operating capital for ongoing development through July 2026, covering the critical period leading up to the FDA's action date on the KETAFREE™ ANDA submission. With revenue now emerging from HOPE clinics and an expanding patient base, the company expects increasing non-dilutive revenue contribution.
Strategic Partnerships Strengthening Commercial Potential
NRx has partnered with Alvogen Pharmaceuticals, leveraging Alvogen's global reach to support development and commercialization of NRX-101 for suicidal bipolar depression. Additional potential indications include non-opioid chronic pain management and treatment of complicated UTIs.
A Pivotal Moment for NRXP Investors
Between its dual-path FDA strategy, real-world data advantages, strong analyst sentiment, and rapidly expanding commercial infrastructure, investors now see NRx Pharmaceuticals reaching an inflection point:
- ✔ FDA-validated ANDA submission for KETAFREE™
- ✔ Fast Track designation for NRX-100
- ✔ Breakthrough Therapy designation for NRX-101
- ✔ $34 analyst price target from D. Boral
- ✔ Manufacturing scale ready for 1M vials/month
- ✔ Real-world ketamine data fueling NDA advancement
- ✔ Nationwide clinic expansion underway
With updated clinical results, strong regulatory momentum, and multiple near-term catalysts, NRx is uniquely positioned to become a leader in next-generation therapies for suicidal depression—a market with urgent unmet need and substantial commercial potential.
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: CorporateAds
0 Comments
Latest on The Californer
- Long Beach: City to Host Doing Business with the City Expo
- Majestic CA Fire & Disaster Safe Haven/VIP Ranch Retreat w/ Extraordinary Water Resources
- A Gift of Books: Local Business Helps Launch Homeless Library in Lancaster
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- DRC Restoration, a Subsidiary of Solid Restoration, Restores Two-Story Home
- iPOP Alum Jacob Batalon Stars in Amazon Prime's "The Wrecking Crew"
- iPOP Alum Olivia Holt Stars in "This Is Not a Test"
- Sellvia Market Enhances Quality Screening for Marketplace Listings
- JiT Home Buyers Strengthens Multi-State Presence as Demand for Flexible Home Selling Solutions Grows
- $3,000,000 Jury Verdict in Police Shooting Case
- Fiz Detailing Launches Professional Car Detailing Services in Fresno, CA
- Transcure Responds to CMS Removal of 285 Inpatient-Only Procedures
- LEVL Launches in over 350 Target Stores Across the East Coast
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- Fritz Coleman's Show "Unassisted Residency" Begins Third Year at El Portal Theatre
- The Good Feet Store Celebrates 25 Years
- Out of the Shadows: JRoc Azael Emerges with Definitive New Album 'Súbele El Volumen"