Trending...
- For Valentine's Day: Treat yourself (and maybe even your sweetheart) to some Not Exactly Love Poems
- CLIKA Built Authenticity at Studio Scale Through a Cultural Lens — Casting Director Paul Sinacore
- Boston Industrial Solutions' Natron® 512N Series UV LED Ink Earns CPSIA Certification
FAYETTEVILLE, Ark. - Californer -- Lineus Medical is now officially registered in the United Kingdom, enabling the company to begin distributing SafeBreak® Vascular within the UK healthcare market. UK registration represents an important step in Lineus Medical's international expansion strategy, further extending access to SafeBreak Vascular for hospitals and clinicians on a global scale. With regulatory requirements in place, Lineus Medical can now work with distribution partners to bring its breakaway IV technology to the UK.
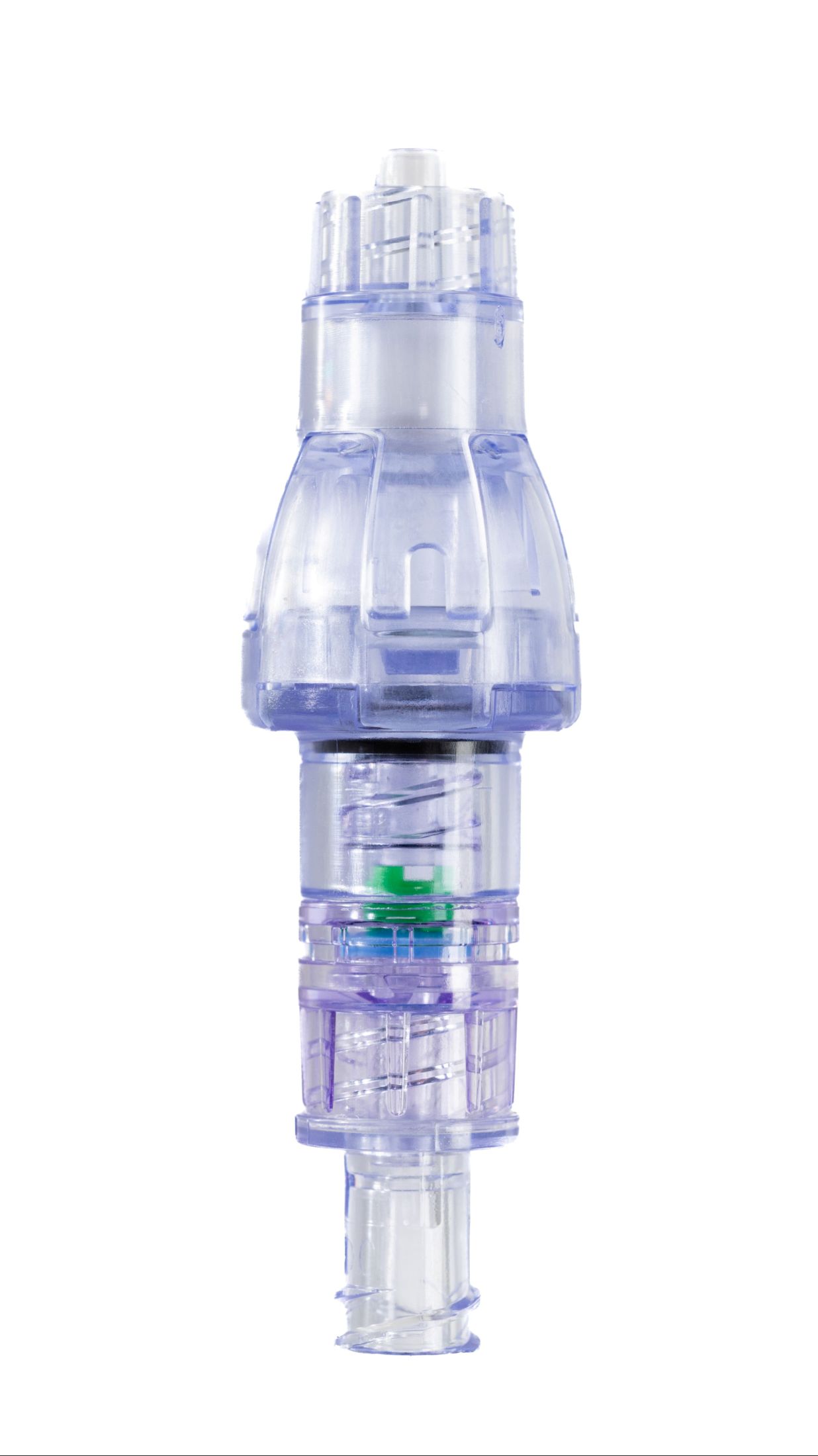
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on The Californer
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on The Californer
- Electric Moon Foundation Receives Grant From Les Paul Foundation
- An Evening of Jazz and R&B with ADAM "AEJAYE" JACKSON at CATALINA JAZZ CLUB, Feb 5
- The Best Online Resource for Getting a Life Insurance Claim Paid
- Eagle Americas Expands Into the Western U.S. With High West Machine Tool
- The Most Informative Resource for Critical Illness Claims
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
- Data on file.
Source: Lineus Medical
0 Comments
Latest on The Californer
- Appliance EMT Partners with Kids Motel Ministry in Metro Atlanta
- CNCPW Divulga Dados de Liquidez do 1º Trimestre: Confirma 100% de Reservas e Atualiza Protocolos de "Saque CNCPW" Contra Fluxos Ilícitos
- Visions Museum Of Textile Art Announces Five New Exhibitions This Winter 2026
- California: Governor Newsom declares Fred Korematsu Day 2026
- Casting Accountability Is Craft: An Intentional Cultural Lens Framework — Paul Sinacore, CSA
- Animal Farm at Theatre School @ North Coast Rep
- Ultimate Print Solutions Prioritizes Customer Experience for Printing Services and Quick Turnaround for Antelope Valley and Los Angeles Markets
- Sleeping Pal Expands U.S. Operations with New Sales Office to Meet Demand for White Noise Machine
- JazzAntiqua Dance & Music Ensemble presents THE STORIES WE TELL
- Biotin, B Vitamin: It's therapeutics, Dr.Abhay Kumar Pati, Physician, Researcher, Phd, Dsc
- Tech Workers Are Escaping "Forever Layoffs" By Becoming Their Own Boss
- All Risk Shield Partners With Watch Duty To Enhance Real-time Wildfire Intelligence And 24-hr Gsoc
- Omniseal Solutions Announces Major Expansion Of Rulon® Fluoropolymers Online Shop
- Glossa Launches Requirements Granularity Dial to Give Teams Control of Requirements Detail
- Long Beach to Host Planning for Housing and HOME Roadshow Open House
- Heritage at South Brunswick Celebrates First Home Closing and Strong Sales Momentum
- California: Governor Newsom, Sacramento State, and Meta advance major redevelopment in downtown Sacramento to create affordable housing for students
- WinkBeds High-Performance Hybrid Mattresses Debut at Sleep Basil Denver With In-Store Comfort Testing
- Tampa Nonprofit Expands Recovery Services for Men in Crisis With New Farm Program in Plant City
- Mesa West Capital Originates $26.9 Million Loan for San Francisco Industrial Property Acquisition