Trending...
- Pacific Harbor Line's Cliatt II Receives Black History Month Trailblazer of the Century Award - 103
- Sellvia Market Enhances Quality Screening for Marketplace Listings
- Transcure Responds to CMS Removal of 285 Inpatient-Only Procedures
H.C. Wainwright Initiates Coverage with $34 Price Target, Citing Paradigm Shift in Depression Treatment. NRx Pharmaceuticals (N A S D A Q: NRXP) $NRXP
MIAMI - Californer -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical company pioneering NMDA-based therapeutics for central nervous system disorders, is emerging as a potential leader in the next generation of depression and suicidality treatments. With its innovative ONE-D (One Day Depression) protocol now launched in Florida and a $40 price target set by H.C. Wainwright, NRXP appears positioned for transformational growth in a rapidly expanding $3.35 billion global market.
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on The Californer
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
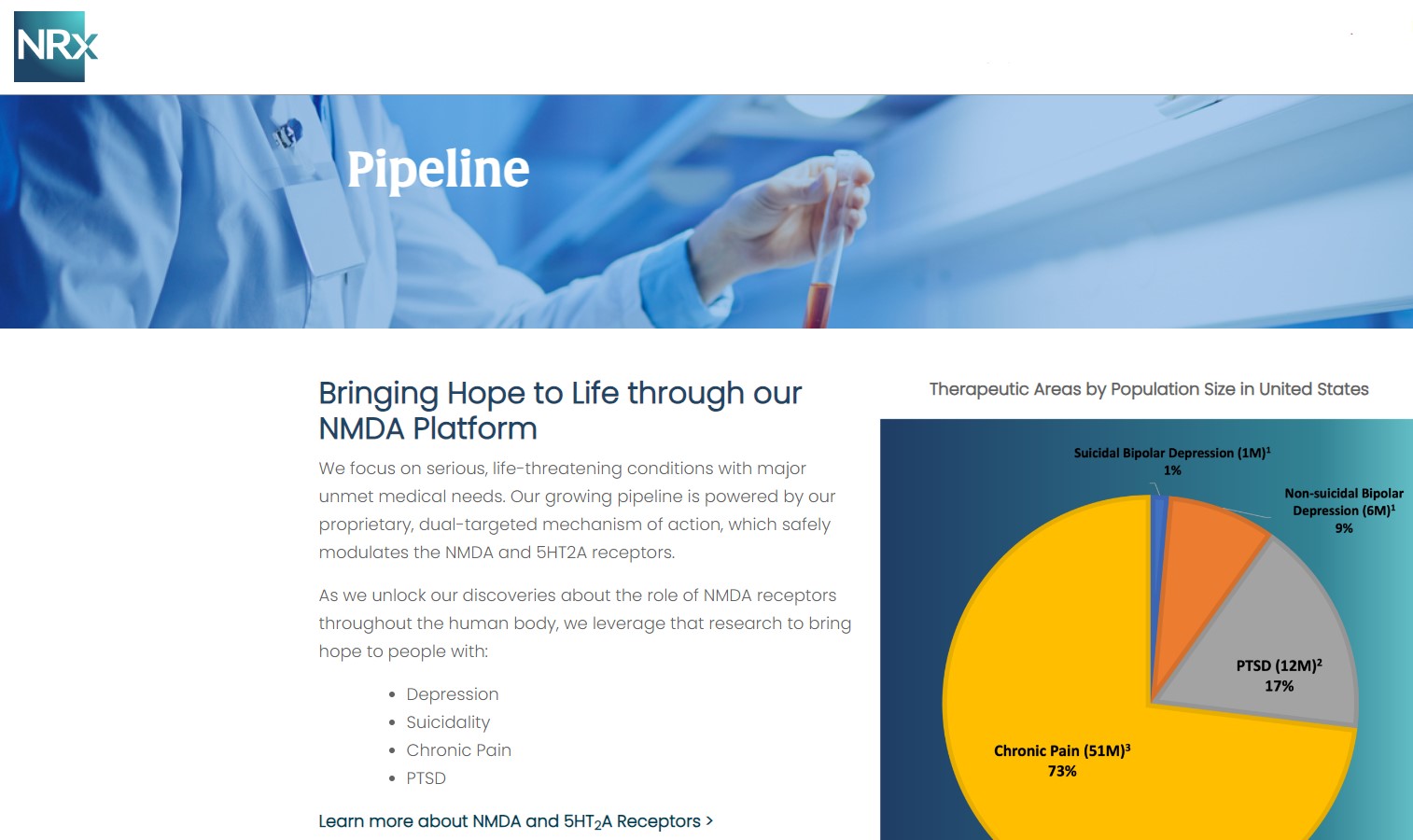
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on The Californer
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
A Major Step Forward: ONE-D Depression Treatment Launches in Florida
NRx Pharmaceuticals has initiated the first-in-Florida rollout of the ONE-D (One Day) Depression Treatment, in partnership with Ampa Health. The innovative protocol has shown breakthrough potential in achieving remission from treatment-resistant depression within a single day—an unprecedented leap forward for millions suffering from chronic depression.
Unlike traditional transcranial magnetic stimulation (TMS) therapies requiring up to 90 sessions over three months, the ONE-D approach combines a single-day TMS treatment with physician-prescribed compounds, including D-cycloserine, an active component of NRx's investigational NRX-101, and lisdexamfetamine. Early clinical data show an 87% response rate and 72% remission rate in nonrandomized studies.
The Ampa device is now available across NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, Florida—with six total locations expected by the end of 2025. This initiative marks one of the first national deployments of the Ampa ONE-D protocol.
More on The Californer
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- From Orientation to IEP Meetings: How AI Is Helping Schools Build Stronger Communities
- Arcuri Group Announces Long‑Term Partnership with WakeMed Health & Hospitals to Deliver Situational Awareness and De‑escalation Training
- California: As Trump tears apart decades of environmental progress, Governor Newsom restores nearly 300,000 acres of habitat and cuts average permitting time to 42 days
- New Rock Music Release "Groove Train"
Breakthrough Therapeutic Portfolio: NRX-101 and KETAFREE™
At the center of NRXP's drug pipeline is NRX-101, an FDA-designated Breakthrough Therapy targeting suicidal, treatment-resistant bipolar depression and chronic pain. The drug represents a potentially lifesaving option for the more than 13 million Americans who seriously consider suicide each year, according to the CDC.
NRXP is also advancing KETAFREE™, a preservative-free IV ketamine formulation, through a refiled Abbreviated New Drug Application (ANDA) with the FDA. This comes as the ketamine market—currently valued at $750 million—is projected to reach $3.35 billion globally by 2034.
Importantly, the FDA has granted approval of NRXP's Suitability Petition for its proposed strength of preservative-free ketamine, clearing the path for potential market entry amid ongoing national shortages of sterile ketamine formulations.
Strategic Expansion Through Dura Medical Acquisition
NRXP recently completed its acquisition of Dura Medical, a revenue-generating and EBITDA-positive network of precision psychiatry clinics along Florida's West Coast. The addition of Dura, alongside pending acquisitions such as Neurospa TMS and Cohen & Associates, positions NRXP to deliver an integrated continuum of mental health care spanning TMS, ketamine therapy, and advanced pharmacologic treatments for PTSD, depression, and chronic pain.
Robust Financial and Partnership Outlook
The company's growth strategy is supported by a $7.8 million debt financing deal with Universal Capital, LLC, aimed at expanding NRXP's HOPE Clinic network. In parallel, NRXP has accepted non-binding potential licensing terms for NRX-100, with the agreement representing over $300 million in milestone payments and tiered double-digit royalties—a major value inflection opportunity for investors.
More on The Californer
- JiT Home Buyers Announces Standardized Nationwide Operating Model to Strengthen Homeowner Experience
- Life Hacks of the Rich and Famous named "Best Self Help Podcast"
- Seth Neblett's Mothership Connected: Focuses on P-Funk's Women as Mothership Celebrates 50 Years
- Passive Appoints Ana Bolčević as Head of Design
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
Moreover, NRXP's partnership with Alvogen Pharmaceuticals supports the ongoing development and commercial readiness of NRX-101, further validating the company's clinical and commercial trajectory.
Analyst Confidence: H.C. Wainwright Issues "Buy" Rating and $40 Price Target
A recent analyst report from H.C. Wainwright & Co. titled "A Paradigm Shift in the Treatment of Depression with Suicidality" initiated coverage on NRx Pharmaceuticals with a Buy rating and a $34 price target. The report underscores NRXP's leadership in transforming how depression and suicidality are treated, leveraging FDA Fast Track and Breakthrough Therapy designations to accelerate commercialization.
"We view NRXP's approach as a paradigm shift in the treatment of depression with suicidality," wrote H.C. Wainwright analysts. "With novel drug candidates, a rapidly expanding clinic network, and strategic licensing opportunities, NRXP is uniquely positioned to capture substantial market share."
About NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
NRx Pharmaceuticals, Inc. is a clinical-stage biopharmaceutical company developing breakthrough therapeutics based on its NMDA receptor modulation platform for the treatment of central nervous system disorders, including suicidal bipolar depression, chronic pain, and PTSD.
Through its partnerships, innovative clinical pipeline, and integrated treatment model, NRXP is setting new standards in precision psychiatry and interventional mental health care.
For More Information:
Company: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com
Website: www.nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on The Californer
- Long Beach: City to Host Doing Business with the City Expo
- Majestic CA Fire & Disaster Safe Haven/VIP Ranch Retreat w/ Extraordinary Water Resources
- A Gift of Books: Local Business Helps Launch Homeless Library in Lancaster
- Purple Heart Recipient Honored by Hall of Fame Son In Viral Tribute Sparking National Conversation on Service Fatherhood, Healing and Legacy
- DRC Restoration, a Subsidiary of Solid Restoration, Restores Two-Story Home
- iPOP Alum Jacob Batalon Stars in Amazon Prime's "The Wrecking Crew"
- iPOP Alum Olivia Holt Stars in "This Is Not a Test"
- Sellvia Market Enhances Quality Screening for Marketplace Listings
- JiT Home Buyers Strengthens Multi-State Presence as Demand for Flexible Home Selling Solutions Grows
- $3,000,000 Jury Verdict in Police Shooting Case
- Fiz Detailing Launches Professional Car Detailing Services in Fresno, CA
- Transcure Responds to CMS Removal of 285 Inpatient-Only Procedures
- LEVL Launches in over 350 Target Stores Across the East Coast
- Amicly Launches as a Safety-First Social App Designed to Help People Build Real, Meaningful Friendships
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- Fritz Coleman's Show "Unassisted Residency" Begins Third Year at El Portal Theatre
- The Good Feet Store Celebrates 25 Years
- Out of the Shadows: JRoc Azael Emerges with Definitive New Album 'Súbele El Volumen"