Trending...
- Demystifying CBD & THC Levels: Ayah Labs' Easy-to-Follow Quantification Guide
- Controversial Vegan Turns Rapper Launches First Song, "Psychopathic Tendencies."
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Has $7.8 Million for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute as Treatment Model and Leading Investigative Site for Suicidal Depression / PTSD
MIAMI - Californer -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
Agreement to Acquire Interest in Cohen and Associates, LLC for Network of Interventional Psychiatry Clinics.
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application with Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
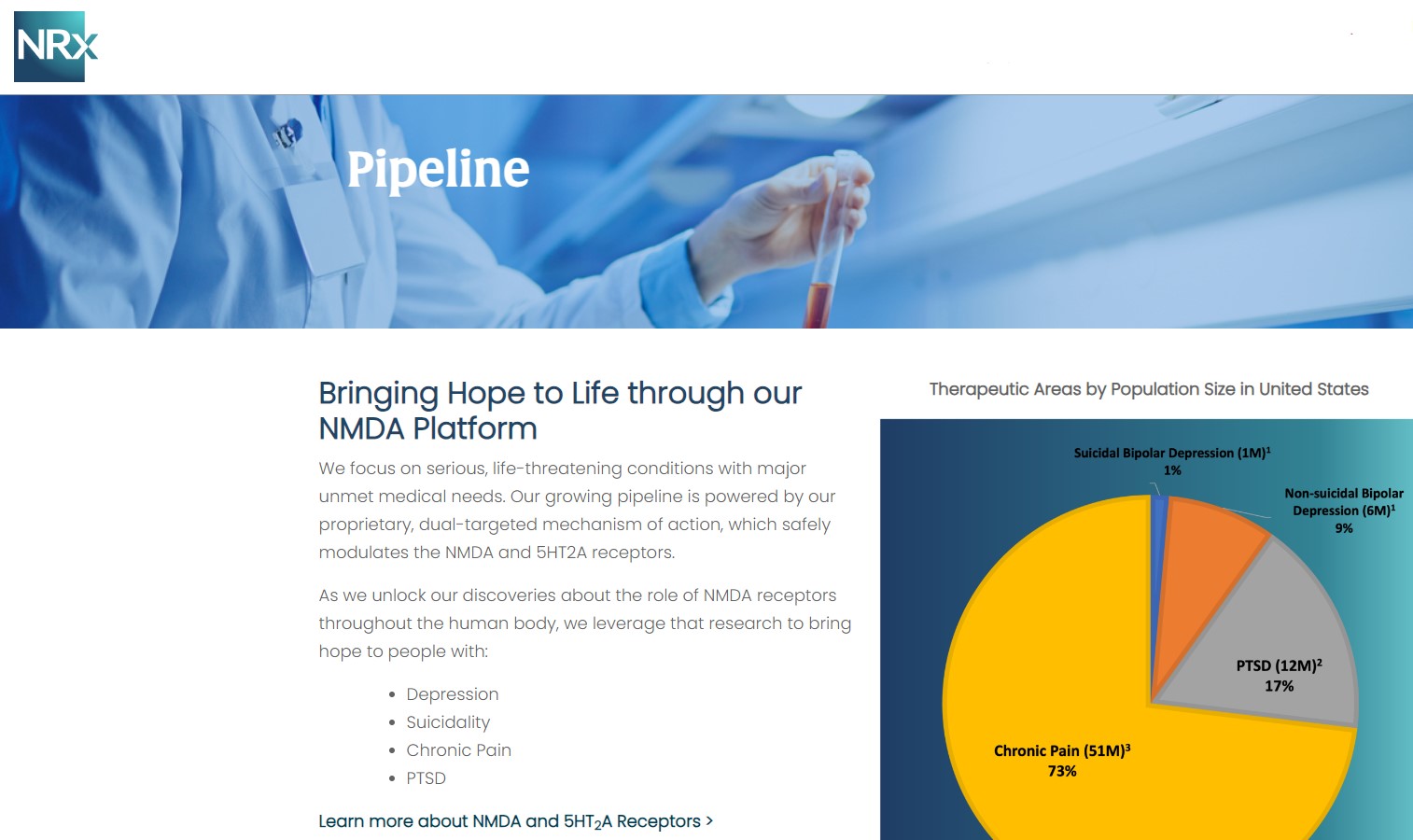
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on The Californer
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
Dura Medical was founded in 2018 to offer a gold-standard, precision approach to treating mental health and chronic pain on the west coast of Florida. The clinics leverage the latest interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), Spravato® and Stellate Ganglion Blocks, augmented by traditional psychiatry and therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders.
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
In June 2025 NRXP filed an Abbreviated New Drug Application with the FDA for a preservative-free preparation of ketamine, demonstrating support for 3 year room temperature stability and sterility. NRXP has similarly filed a patent on its preservative-free process, in light of prior art that suggested BZT was required for long term stability and sterility. NRXP has instituted US-based high volume manufacture, while it awaits generic approval. The Company is additionally seeking a labeled indication for the use of ketamine to treat suicidal depression through the recently-announced FDA Commissioner's National Priority Voucher Program.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
More on The Californer
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Cohen is one of the premier Interventional Psychiatry clinics in the region. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, Transcranial Magnetic Stimulation ("TMS") as well as medication management. NRXP stated that this acquisition should be immediately accretive to revenue and EBITDA.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Strategic Investor Relations Partnership with astr partners
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
Agreement to Acquire Interest in Cohen and Associates, LLC for Network of Interventional Psychiatry Clinics.
Applied for New Commissioner's National Priority Vouchers (CNPV) for NRX-100 With Anticipated Decisions on Drug Approval by Year-End.
Application Under CNPV Program is Accretive to Already-Filed Abbreviated New Drug Application with Proprietary Formulation Under Priority Review Request.
Abbreviated New Drug Application Filed for Preservative-Free IV Ketamine.
Ketamine Market Estimated at $750 Million and Projected to Reach $3.35 Billion Globally in 2034.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on The Californer
- NEW Luxury Single-Family Homes Coming Soon to Manalapan - Pre-Qualify Today for Priority Appointments
- Introducing The 'A Hot Set' 2026 Media Kit
- Dominic Pace Returns to the NCIS Franchise With Guest Role on NCIS: Origins
- Anderson Periodontal Wellness Attends 5th Joint Congress for Ceramic Implantology
- Vallejo Realtor Bruno Versaci Continues to Lead the Market
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA)
On August 8th NRXP announced it has received final clearance and approval from the Florida Agency for Health Care Administration (AHCA) to proceed closing of its Dura Medical LLC acquisition, in connection with its change of ownership applications, a key regulatory step for closing. Dura is revenue generating and EBITDA positive.
Dura Medical was founded in 2018 to offer a gold-standard, precision approach to treating mental health and chronic pain on the west coast of Florida. The clinics leverage the latest interventional psychiatry procedures, including Ketamine Infusion Therapy, Transcranial Magnetic Stimulation (TMS), Spravato® and Stellate Ganglion Blocks, augmented by traditional psychiatry and therapy to provide a full continuum of care for people with depression, suicidality, PTSD, anxiety, and related disorders.
Request to the Food and Drug Administration Seeking Removal of Benzethonium Chloride from Ketamine Products to Favor a Switch to Better Options
On August 4th NRXP announced its actions with the US Food and Drug Administration (FDA), seeking the removal of Benzethonium Chloride from ketamine sold in the United States. Benzethonium Chloride (BZT) is a preservative that is not Generally Recognized as Safe (GRAS) by the FDA for parenteral products and not Generally Recognized as Safe and Effective (GRASE) for topical products. The FDA no longer allows BZT to be used in hand cleansers and topical antiseptics and other products on the market today for safety concerns. The ketamine versions made by NRXP is BZT free and therefore presents a safer and superior option.
In June 2025 NRXP filed an Abbreviated New Drug Application with the FDA for a preservative-free preparation of ketamine, demonstrating support for 3 year room temperature stability and sterility. NRXP has similarly filed a patent on its preservative-free process, in light of prior art that suggested BZT was required for long term stability and sterility. NRXP has instituted US-based high volume manufacture, while it awaits generic approval. The Company is additionally seeking a labeled indication for the use of ketamine to treat suicidal depression through the recently-announced FDA Commissioner's National Priority Voucher Program.
Agreement to Acquire Interest in Cohen and Associates, LLC for HOPE's Network of Interventional Psychiatry Clinics
More on The Californer
- California: Governor Newsom's SAFE Task Force partners with Long Beach to address encampments
- UK Financial Ltd Completes Full Ecosystem Conversion With Three New ERC-3643 SEC-Ready Tokens As MCAT Deadline Closes Tonight
- Governor Newsom helps provide more than a thousand Californians with homes
- AI Real Estate Company Quietly Building a National Powerhouse: reAlpha Tech Corp. (N A S D A Q: AIRE)
- Inboox.ai Surpasses 1,000 Users After Public Launch of Email Inspiration and AI Analysis Platform
On June 26th NRXP announced the signing of a binding Letter of Intent to purchase a 49% interest in Cohen and Associates, LLC. Cohen is expected to serve as a foundational clinic for NRXP in the Sarasota-Bradenton region of western Florida.
Cohen is one of the premier Interventional Psychiatry clinics in the region. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, Transcranial Magnetic Stimulation ("TMS") as well as medication management. NRXP stated that this acquisition should be immediately accretive to revenue and EBITDA.
Filing of Commissioner's National Priority Voucher Application for Intravenous Ketamine (NRX-100)
On June 23rd NRXP announced filing for the newly-announced FDA Commissioner's National Priority Voucher program on behalf of NRX-100, its patent-pending, preservative-free formulation of ketamine for intravenous use.
On June 17, 2025, FDA Commissioner Marty Makary, MD, MPH announced a new approval pathway, the Commissioner's National Priority Voucher (CPNV)1, for approval of drugs to enhance the health interests of Americans. Previously, on May 25 he identified psychedelic drugs for treatment of suicidal depression and PTSD as a national priority.2 The new voucher may be redeemed by drug developers to participate in a Commissioner-led program that shortens its review time from approximately 10-12 months to 1-2 months following a sponsor's final drug application submission.
To qualify for the CNPV, sponsors must submit the chemistry, manufacturing, controls (CMC) portion of the application and the draft labeling at least 60 days before submitting the final application. NRXP has already submitted the CMC portion for NRX-100 and received FDA feedback.
Strategic Investor Relations Partnership with astr partners
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on The Californer
- Melospeech updates proprietary MeloSuite platform to improve service delivery and response time
- GreenSTOP LA Unveils World's First Fully Automated Retail Store Powered by Revolutionary 4-Person Simultaneous Vending Technology
- Crypto Corner Shop, Built by Afghan Refugee, Set to Launch Soon!
- Verb™ Presents Features Vanguard Personalized Indexing: Utilizing Advanced Tax-Loss Harvesting Technology
- California: Newsom blasts CDC panel after vote to end universal newborn hepatitis B vaccinations
- Saluting a record year for CHP cadets, California's next generation of law enforcement officers
- Inboox.ai Launches AI-Powered Library of One Million Real Ecommerce Emails for Marketers
- California: Governor Newsom meets with congressional leaders to press for long-delayed LA wildfire aid
- California: Governor, First Partner statement on the passing of Frank Gehry
- Zacuto Group Brokers Sale of 1936 Mateo Street in Downtown Los Angeles
- A Holiday Classic Reborn: Lisa Dawn Miller Reimagines Her Father's Holiday Hit
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- Awepra Launches the Awepra Shop: A New Luxury Marketplace for Curated Excellence
- Ventura College Nursing Program Ranked #1 for NCLEX-RN Pass Rates
- Cosmetic Injectables Center Medspa Expands Advanced Laser Treatment Services in Sherman Oaks
- Long Beach Parks, Recreation and Marine to Host Free, Family-Friendly Holiday Events This December
- San Francisco Farm Bureau to Host Second Annual Farm to Fork Dinner & Awards Ceremony
- Free Kids SoccerFit Curriculum Download Available This Holiday, Released by Exercise Daily Media Lab
- 6 Holiday Looks That Scream "Old Money" But Cost Less Than Your Christmas Tree
- Affordable Luxury Jewellery Gifts for Christmas 2025: How Ermoleve Elevates Everyday Style