Trending...
- Grammy award-winning Cuban-Canadian artist Alex Cuba releases his 11th studio album, "Indole"
- California: Crime is down in San Francisco, key law enforcement partnerships yield successful results
- $73.6M Pipeline, $10M Crypto Play & Legal Firepower: Why Investors Are Watching Cycurion (N A S D A Q: CYCU) Like a Hawk
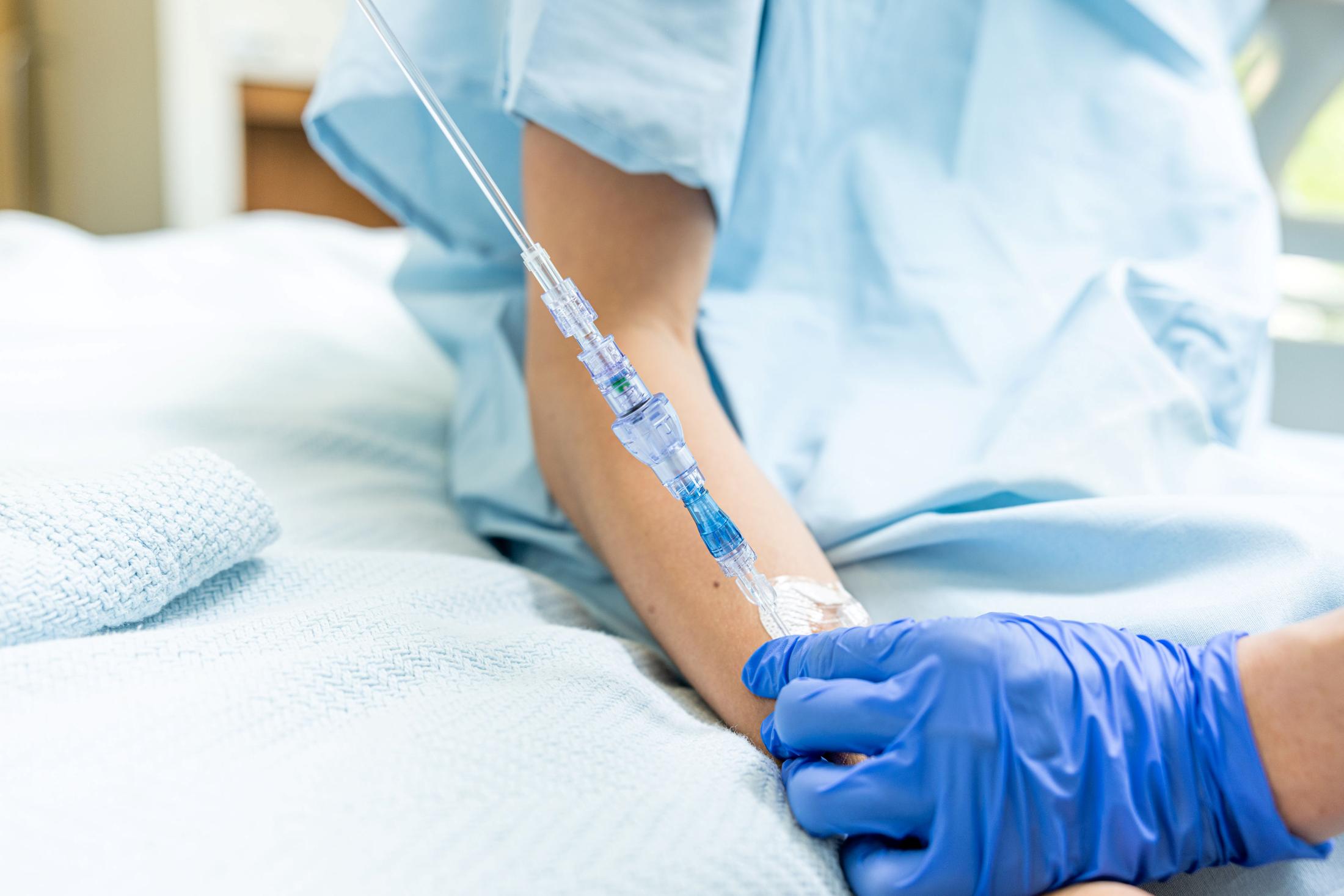
FAYETTEVILLE, Ark. - Californer -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on The Californer
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on The Californer
- Luxury Hospitality Advisors Launches Realtor Training Based on Five Star Hotel Service Training
- Swidget Launches Luminance™ to Help Schools Achieve Alyssa's Law Compliance
- Farther's Michael Lee Named One of InvestmentNews' Rising Stars of 2025
- Growing Demand for EVA Mats Signals Shift in Car Interior Market
- 4th Annual Holidays In The Village, A European Marketplace
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
0 Comments
Latest on The Californer
- California strengthens its pipeline for good-paying jobs, providing $25 million to train more than 22,000 workers through apprenticeship programs
- Why Voting "No on Prop 50" Makes Sense
- Hollywood In Pixels Celebrates the 8th Annual Silver Pixel Awards and Announces 2025 Campaign Pixel Winners Los Angeles, CA — Oct
- While the Trump administration cuts programs to fight hate crimes, California is taking action every day
- The AI Journal Publishes Groundbreaking Article
- Physician Calls for States Nationwide to Ensure ADA Compliance in Independent Commissions
- MEDIA ADVISORY - Strengthening Children's Mental Health Across New Jersey
- Culinage Launches to Preserve Family Recipes and the Stories Behind Them
- NumberSquad Launches Year‑Round Tax Planning Package for Small Businesses and the Self‑Employed
- GlexScale launches a unified model for sustainable SaaS expansion across EMEA
- SwagHer Society Launches to Help Black Women Be Seen and Supported
- Why Hybrid Work Makes Mileage Tracking Harder
- Faces of Rap Mothers Announces Open Submissions for Upcoming Volumes
- Countrywide Rental Elevates Adger's Hygiene Standards Through Expanded Portable Restroom Solutions
- Why Philadelphia Homeowners Should Ditch Oil for Natural Gas
- BeatsToRapOn launches Verified Music Promotion Marketplace powered by AI Agents
- Who plays xs in the flash and which episodes has her? "Schway!"
- Frankie Blair Stars in "Disturbing Intentions: Good vs. Evil," Coming to Amazon Prime & Tubi Oct. 24
- Zero-Trust Architecture: NJTRX Addresses 60% of U.S. Investors' Custody Security Concerns
- White House to fire explosive artillery over major roadway in Southern California, I-5 to be temporarily shut down on Saturday due to life safety risk