Trending...
- California: Governor Newsom, Superintendent Thurmond announce over $618 million to support another 458 community schools
- $4.3 Million Patent Application Waiver Fee Granted by FDA on New Drug Application Fee for Treatment Addressing Suicidal Depression & PTSD: NRX Pharma
- xREnergy up as much as +3,094,634% on first day listed on the XRP Ledger. Ticker : $XRE
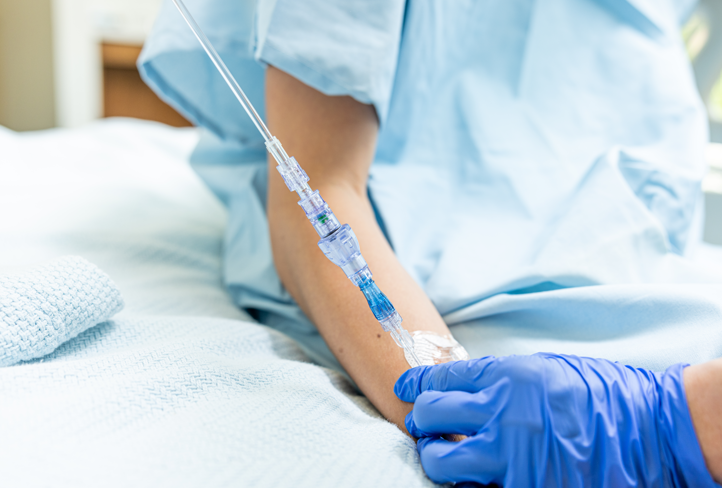
FAYETTEVILLE, Ark. - Californer -- Health Canada has issued a licence on Lineus Medical's flag ship product, SafeBreak® Vascular, for use on all vascular access lines including peripheral IV catheters, peripherally inserted central catheters, central venous catheters, midlines, IV ports, and interosseous vascular access devices on patients two weeks of age and up. This licence makes Lineus Medical the first company in the world to obtain authorization for the use of a break-away device on all vascular access lines and for both pediatric and adult patients in a country.
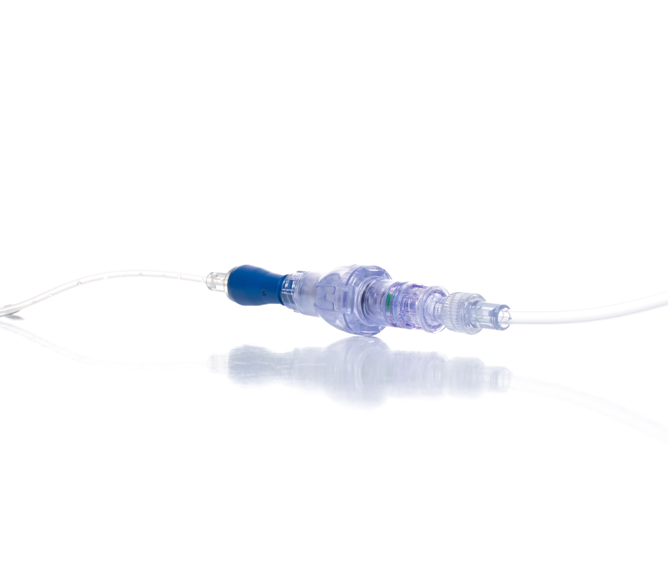
SafeBreak Vascular is the only breakaway device for IV lines proven to reduce IV complications.1 When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. The separated device also causes the IV pump to alarm, notifying the nursing staff to check on the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. It's a win-win-win. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on The Californer
Will Armstrong, COO of Lineus Medical stated, "This has been a year of growth for the company. This past May, SafeBreak received pediatric clearance for peripheral IVs from the US Food and Drug Administration, and now we are licenced on all lines for children and adults in Canada. We look forward to making SafeBreak available to every hospital and the over 38 million people living in Canada."
Vance Clement, CEO of Lineus Medical, stated, "Lineus Medical is the first company to obtain licence in a country on the full array of vascular lines across pediatric to adult aged patients. Not only are we expanding into every clinical setting including, Emergency Medical Services, veterinary care, and acute care hospitals systems, we are expanding internationally. Every patient, no matter where they live in the world, deserves a SafeBreak on their IV line."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Facebook or LinkedIn.
More on The Californer
1. Data on File
MKG-0230 Rev 00
SafeBreak Vascular is the only breakaway device for IV lines proven to reduce IV complications.1 When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. The separated device also causes the IV pump to alarm, notifying the nursing staff to check on the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient's infusion is restarted. It's a win-win-win. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on The Californer
- Bennett Awards Designs Two Cohesive Custom Awards for 2025 Sci-Tech Academy Awards
- Poll Finds Overwhelming Opposition to Keeping Big Cats as Pets
- SM Telecom Expands AT&T Partnership to Deliver Cutting-Edge 5G+ Wireless Solutions New Collab Brings AT&T's Advanced 5G+ Technology to Cellphone
- Governor Newsom proclaims Older Californians Month
- Groundbreaking Launches 154-Acre Los Cerritos Wetlands Restoration in Long Beach – Single Largest Increase in Open Space in Long Beach in Decades
Will Armstrong, COO of Lineus Medical stated, "This has been a year of growth for the company. This past May, SafeBreak received pediatric clearance for peripheral IVs from the US Food and Drug Administration, and now we are licenced on all lines for children and adults in Canada. We look forward to making SafeBreak available to every hospital and the over 38 million people living in Canada."
Vance Clement, CEO of Lineus Medical, stated, "Lineus Medical is the first company to obtain licence in a country on the full array of vascular lines across pediatric to adult aged patients. Not only are we expanding into every clinical setting including, Emergency Medical Services, veterinary care, and acute care hospitals systems, we are expanding internationally. Every patient, no matter where they live in the world, deserves a SafeBreak on their IV line."
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Facebook or LinkedIn.
More on The Californer
- Don Barnhart Drops Unapologetically Hilarious Comedy Special "You Do You," On Open Bar Network
- Cygnet Theatre Announces The Cast and Creative Team of Rodgers and Hammerstein's Oklahoma!
- Shon Garage Door Repair Expands Trusted Services to San Diego, CA
- Nicky Dare Honors Indonesian Heritage at VAPI–Valley Asian and Pacific Islanders Cultural Festival
- California launches new AI-powered chatbot that provides wildfire resources in 70 languages
1. Data on File
MKG-0230 Rev 00
Source: Lineus Medical
0 Comments
Latest on The Californer
- Gravity to Bring 5-Minute EV Charging to 8 Sites Across Greater LA
- California: Governor Newsom issues statement on Pope Leo XIV, the first American Pope
- New poll shows high rates of employee burnout amid concerns over politics and personal finances
- Tessellations Appoints Luthern Williams as Head of School
- Aureli Construction Sets the Standard for Seamless Home Additions in Greater Boston
- Psychological Thriller "Killing Off Connor" To Open 34th IFS Film Fest After 12-years In Post
- Harvest Properties Acquires Two San Francisco Bay Area Self Storage Facilities for $44.2 Million
- California businesses in near-universal compliance with prohibition of intoxicating hemp products harmful to youth
- California: Governor Newsom announces upgrades to 21 state fish hatcheries to boost salmon populations
- Solaris Energy Infrastructure, Inc. (SEI) Investors Who Lost Money Have Opportunity to Lead Securities Fraud Lawsuit
- Risk Rater, Threat Assessment App, gives Users the Same Threat Evaluation as the Rich and Powerful
- Is it Really True That Tariffs Will Raise Car Insurance Rates?
- ScreenPoints Puts Film Investors in the Credits—and in the Money With New FinTech Platform
- Coastal Business Systems Wraps Up Successful 2025 Tech Show in Redding
- AdOcto Turns AirBnBs Into High-Impact Advertising Channels
- Zefr Announces Launch of Pre-Screen Brand Safety Solution for Google's Search Partner Network (SPN)
- Pathways to Adulthood Conference May 17 at Melville Marriott Honoring NYS Assembly Member Jodi Giglio, Suffolk County Legislator Nick Caracappa
- Adster Techologies awarded US Patent for breakthrough innovation in reducing latency in Ad Serving
- Flexi-View Lending Closes $5.05 Million Residential Acquisition Loan in Billings, Montana
- Robert Fabbio Inducted into the Austin Technology Council Hall of Fame